pharmachemist
hi...............
Tuesday, October 15, 2019
Sunday, July 21, 2019
Story of Valsartan
The story of Valsartan and Nitroamine impurities
Hoping that our all pharma fellow employees will know the story and this could create knowledge
On July 17, 2018 and July 18, 2018, respectively, the EMA and the FDA reported a major issue regarding the detection of a genotoxic impurity, NDMA (N-nitrosodimethylamine), and subsequently NDEA (N-nitrosodiethylamine), in current lots of valsartan,1 an API used to manufacture generic angiotensin receptor blockers (ARBs). The API at issue was supplied by two Chinese companies, Zhejiang Huahai and Zhejiang Tianyu, and was later discovered in API manufactured by Indian manufacturer Hetero Labs. Both NDMA and NDEA are considered genotoxic compounds as they contain a nitroso group, which is a gene mutating group. This concern has spread across the EU, Canada, and the U.S., and is expanding to other regions as well.
NDMA is found in nature and food, including in some water supplies, in very low levels.2 Consuming up to 96 nanograms NDMA/day is considered reasonably safe for human ingestion.3
Probable Origin Of NDMA/NDEA And Its Impact On Other APIs
Most ARBs have a chemical structure that includes a tetrazole group. It appears that tetrazole ring formations, coupled with certain manufacturing conditions using the solvent N, N-Dimethylformamide, aka DMF, gave rise to this class of impurities in drug substance intermediates used in sartans. Other tetrazole ring formations make up APIs like candesartan, losartan, irbesartan, and olmesartan. The impurity may also roll over to other compounds normally known as “zoles,” such as omeprazole, etc.4,5,6
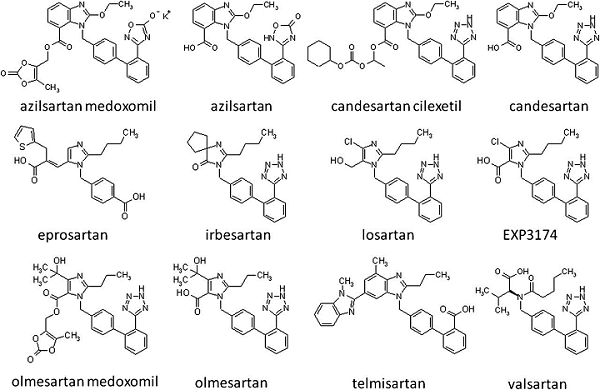
Figure 1: Structure of sartans
The nitroso impurity may well roll over to other tetrazole rings containing such molecules as cefonacid, ceforanide, cefoperazone, cefmetazole, colostazole, latamoxef, parnlukast, and pemiroplast, to name a few.
Regulatory Assessment Of The Process Change
It is not known whether the solvent was newly introduced into the manufacturing process during the process changes that occurred in 2011-2012. However, as reported by various agencies, these impurities started to appear in the product after the change in the process; hence, it is likely the solvent was newly introduced in the synthesis, and the following assessment is based on that assumption. On the contrary, however, Huahai U.S. has issued a statement indicating that the impurity appears only in recent lots.7
European Perspective
According to the-then-official European Directorate for the Quality of Medicines (EDQM) guideline for revisions to an approved certificate of suitability (CEP)8 at the time of the change submitted by the manufacturer, “MAJ3 Substantial change to the manufacturing process/addition of an alternative manufacturing process for a starting material, intermediate or final substance likely to change the qualitative and/or quantitative impurity profile (e.g., new reagents, solvents, materials are introduced in the synthesis).”
This clearly indicates that when a new solvent is introduced, it is considered a major change and will be assessed accordingly by the respective agency in line with the requirements for assessment of genotoxic impurities. However, the manufacturer missed reporting the generation of the new impurity (NDMA/NDEA, genotoxic impurity) in the revised process.
A revised EDQM guideline for CEP revisions, shown in Table 1, was published in July 2014.9
Table 1: Assessment of Revision in CEP Application
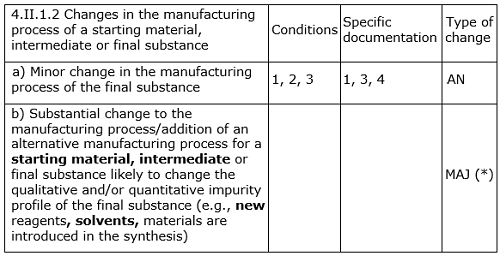
It is again very clearly indicated in this and other relevant guidelines that if the proposed change has significant impact on the qualitative and/or quantitative impurity profile, then the levels of known, unknown, and total impurities found in the post-change batches should be assessed/compared critically with respect to with those of pre-change batches.
EDQM subsequently (September 2018, with implementation date of January 2019) changed10 the above revision guideline to include evaluation for genotoxic impurities as well.
Interestingly, the manufacturer, Zhejiang Huahai, stated that the change was implemented in 2012 after approval by relevant regulatory authorities,11 without clarifying which authorities approved the process change.
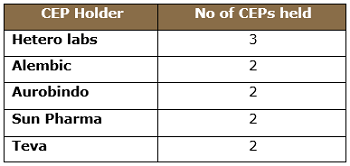
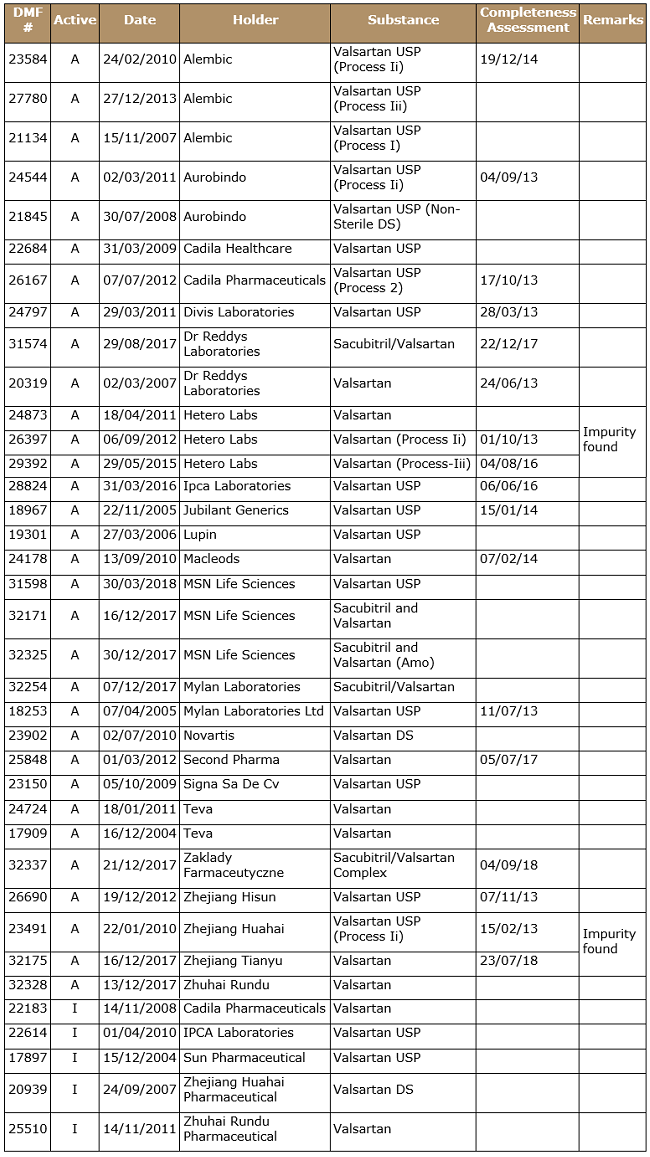
Approximately 28 CEPs for valsartan are approved by EDQM, as tabulated below, with various statuses found in the EDQM CEP database. Five companies hold more than one CEP, meaning they are using more than one manufacturing process.
Table 2: Major Valsartan CEP Holders
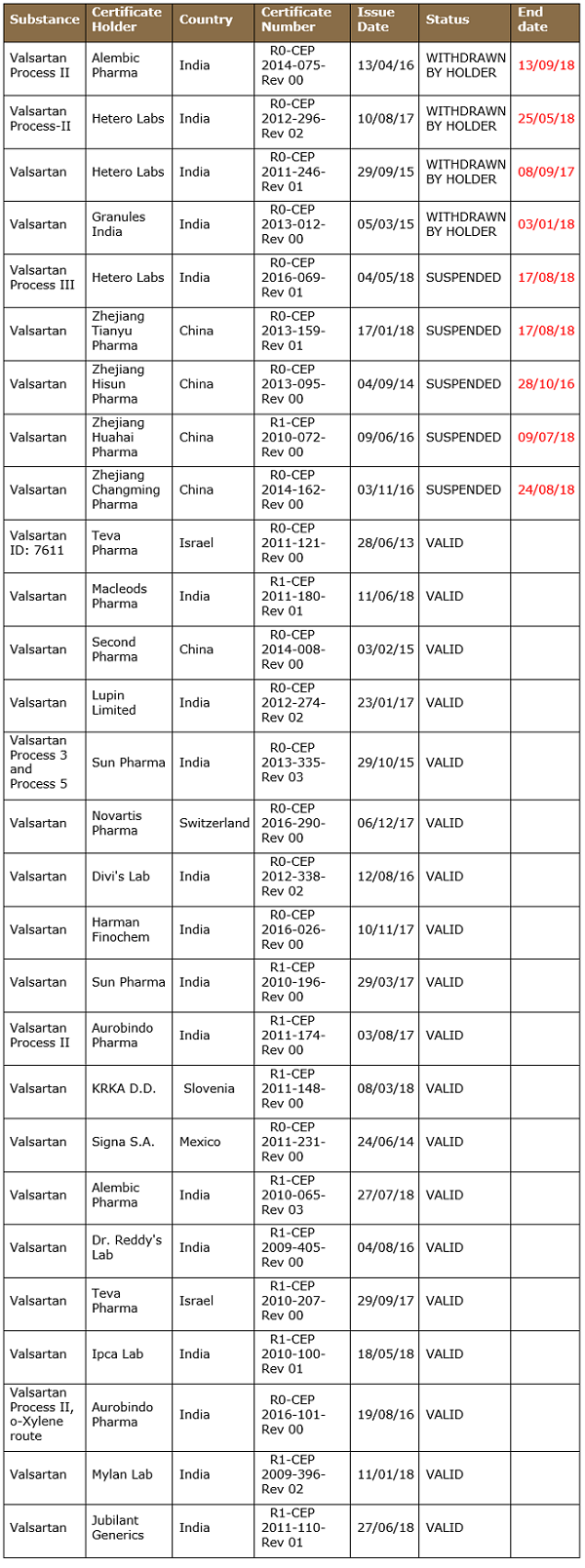
EDQM has suspended four CEPs after the detection of NDMA. Interestingly, Indian manufacturer Alembic Limited voluntarily withdrew its CEP in September 2018, following the nitroso impurity episode.
Table 3: List of Valsartan CEPs as of Sept. 17, 2018
U.S. Perspective
The FDA has different assessment criteria and methodology than the EU/EDQM. The guidance Changes to an Approved NDA or ANDA12 applies to changes made throughout the drug substance manufacturing process, i.e., from the starting material through the final drug substance.
Changes in key starting material manufacturing processes are left to the evaluation of the master file holder and, based on the evaluation, reporting criteria, and related documentation, shall be submitted in master files.
In section VII.B.5, the guidance indicates that “Changes in the synthesis or manufacture of the drug substance that may affect its impurity profile and/or the physical chemical or biological properties.”
The assessment of such changes should be done based on sections IV.A.1 and IV.A.2:
1. Conformance to Specifications
“Conformance to a specification means that the material, when tested according to the analytical procedures listed in the specification, will meet the listed acceptance criteria.”
2. Additional Testing
Evaluation of changes in the impurity or degradant profile could first involve profiling using appropriate chromatographic techniques and then, depending on the observed changes in the impurity profile, toxicology tests to qualify a new impurity or degradant or to qualify an impurity that is above a previously qualified level.
This guideline is enough to alert manufacturers to evaluate and assess the new impurities that may form during the changed process and their subsequent impact on the marketed substance and product.
In the new draft guidance Postapproval Changes to Drug Substances13 Section VIII.B (Changes in Route of Synthesis of One or More Steps) states:
In general, changes in route of synthesis are considered to have a moderate to high potential to adversely affect the impurity profile of the drug substance. The manufacturing process should be validated using the new route of synthesis. Impurity carryover studies and spike/purge studies should be conducted as appropriate. Control of mutagenic impurities in or expected to be in the final drug substance should be evaluated according to ICH M7 (section 4.1).
This new guideline explicitly mentions impact assessment as per ICH M7 for genotoxic impurities.
Table 4: List of USDMF as on Sep. 17, 2018
Assessment
Nitroso impurities are very well studied and documented in food industry and drinking water specifications worldwide. However, their origin and impact were not studied or extrapolated in pharmaceutical substance manufacturing processes.
I have reason to believe that all the submission holders have been updating their regulatory submissions according to the effective guidelines and that regulators have acted according to the prevalent knowledge database at their end.
As a recent article rightly pointed out,14 pharmaceutical substances can’t be and probably will not be analyzed for all the probable carcinogens on a regular basis because of limitations with pharmacopoeias, regulatory authorities, and the industry.
However, this unfortunate incident, which is still being extended to other ARBs (losartan, and irbesartan), forces us to look beyond the mere guidelines and processes to the experience of other industries, in this case nitroso impurities in the food and water industries.
This case may benefit some manufacturers that claim their products are “nitroso impurity free,” as well as analytical laboratories that have validated methods for analyses of nitroso impurities in drug substances.
To avoid cases similar to this in the future, the pharmaceutical industry may have to consider adopting a collaborative approach during the research, development, and manufacture of drug substances to absorb the knowledge available in other domains.
Notes:
- Valsartan is an angiotensin II receptor blocker (ARB) that treats high blood pressure and heart failure.
Wednesday, January 23, 2019
Thursday, March 30, 2017
Thursday, March 23, 2017
Thursday, February 26, 2015
HPLC GURAD COLUMNS
HPLC Guards are really very useful accessory to protect guards so that the life time of the column and commercial viability for the organization is mutual benefit for the analyst and organization, here are some typical are like.
Guard columns, set between the injector and an analytical column, are used to protect analytical columns from chemical impurities in samples. We have two types of guard columns : cartridge type which can be changed easily by hand, and packed type, which are packed in stainless cartridge like analytical columns.
How to choose guard column is introduced below. “Cartridge guard column E” is the most useful and is recommended as a high performance guard column.
In order to obtain maximum protection , it is recommended to use a guard column that contains the same packing material as the analytical column.
Product list of Guard columns are displayed with analytical columns. Please go to the product page to which the Guard columns are connecte
Monday, February 24, 2014
Water purification
Water purification
From Wikipedia, the free encyclopedia
This article is about large scale, municipal water purification. For small scale and home water purification, see water filter. For portable/emergency water purification, see portable water purification. For industrial water purification, see deionized water. For distilled water, see distilled water.
Control room and schematics of the water purification plant to Lac de Bret, Switzerland
The purification process of water may reduce the concentration of particulate matter including suspended particles, parasites, bacteria, algae, viruses, fungi; and a range of dissolved and particulate material derived from the surfaces that water may have made contact with after falling as rain.
The standards for drinking water quality are typically set by governments or by international standards. These standards will typically set minimum and maximum concentrations of contaminants for the use that is to be made of the water.
It is not possible to tell whether water is of an appropriate quality by visual examination. Simple procedures such as boiling or the use of a household activated carbon filter are not sufficient for treating all the possible contaminants that may be present in water from an unknown source. Even natural spring water – considered safe for all practical purposes in the 19th century – must now be tested before determining what kind of treatment, if any, is needed. Chemical and microbiological analysis, while expensive, are the only way to obtain the information necessary for deciding on the appropriate method of purification.
According to a 2007 World Health Organization (WHO) report, 1.1 billion people lack access to an improved drinking water supply, 88 percent of the 4 billion annual cases of diarrheal disease are attributed to unsafe water and inadequate sanitation and hygiene, and 1.8 million people die from diarrheal diseases each year. The WHO estimates that 94 percent of these diarrheal cases are preventable through modifications to the environment, including access to safe water.[1] Simple techniques for treating water at home, such as chlorination, filters, and solar disinfection, and storing it in safe containers could save a huge number of lives each year.[2] Reducing deaths from waterborne diseases is a major public health goal in developing countries.
Contents
- 1 History
- 2 Sources of water
- 3 Treatment
- 4 Other water purification techniques
- 5 Safety and controversies
- 6 See also
- 7 References
- 8 Further reading
- 9 External links
History
The first experiments into water filtration were made in the 17th century. Sir Francis Bacon attempted to desalinate sea water by passing the flow through a sand filter. Although his experiment didn't succeed, it marked the beginning of a new interest in the field. Fathers of microscopy, Antonie van Leeuwenhoek and Robert Hooke, used the newly invented microscope to observe for the first time small material particles that lay suspended in the water, laying the groundwork for the future understanding of waterborne pathogens.[3]Sand filter
The first documented use of sand filters to purify the water supply dates to 1804, when the owner of a bleachery in Paisley, Scotland, John Gibb, installed an experimental filter, selling his unwanted surplus to the public.[4] This method was refined in the following two decades by engineers working for private water companies, and it culminated in the first treated public water supply in the world, installed by engineer James Simpson for the Chelsea Waterworks Company in London in 1829.[5] This installation provided filtered water for every resident of the area, and the network design was widely copied throughout the United Kingdom in the ensuing decades.The practice of water treatment soon became mainstream, and the virtues of the system were made starkly apparent after the investigations of the physician John Snow during the 1854 Broad Street cholera outbreak. Snow was sceptical of the then-dominant miasma theory that stated that diseases were caused by noxious "bad airs". Although the germ theory of disease had not yet been developed, Snow's observations led him to discount the prevailing theory. His 1855 essay On the Mode of Communication of Cholera conclusively demonstrated the role of the water supply in spreading the cholera epidemic in Soho,[6][7] with the use of a dot distribution map and statistical proof to illustrate the connection between the quality of the water source and cholera cases. His data convinced the local council to disable the water pump, which promptly ended the outbreak.
The Metropolis Water Act introduced the regulation of the water supply companies in London, including minimum standards of water quality for the first time. The Act "made provision for securing the supply to the Metropolis of pure and wholesome water", and required that all water be "effectually filtered" from 31 December 1855.[8] This was followed up with legislation for the mandatory inspection of water quality, including comprehensive chemical analyses, in 1858. This legislation set a worldwide precedent for similar state public health interventions across Europe. The Metropolitan Commission of Sewers was formed at the same time, water filtration was adopted throughout the country, and new water intakes on the Thames were established above Teddington Lock. Automatic pressure filters, where the water is forced under pressure through the filtration system, were innovated in 1899 in England.[4]
Water chlorination
John Snow was the first to successfully use chlorine to disinfect the water supply in Soho that had helped spread the cholera outbreak. William Soper also used chlorinated lime to treat the sewage produced by typhoid patients in 1879.In a paper published in 1894, Moritz Traube formally proposed the addition of chloride of lime (calcium hypochlorite) to water to render it “germ-free.” Two other investigators confirmed Traube’s findings and published their papers in 1895.[9] Early attempts at implementing water chlorination at a water treatment plant were made in 1893 in Hamburg, Germany and in 1897 the city of Maidstone England was the first to have its entire water supply treated with chlorine.[10]
Permanent water chlorination began in 1905, when a faulty slow sand filter and a contaminated water supply led to a serious typhoid fever epidemic in Lincoln, England.[11] Dr. Alexander Cruickshank Houston used chlorination of the water to stem the epidemic. His installation fed a concentrated solution of chloride of lime to the water being treated. The chlorination of the water supply helped stop the epidemic and as a precaution, the chlorination was continued until 1911 when a new water supply was instituted.[12]
The technique of purification of drinking water by use of compressed liquefied chlorine gas was developed by a British officer in the Indian Medical Service, Vincent B. Nesfield, in 1903. According to his own account, "It occurred to me that chlorine gas might be found satisfactory ... if suitable means could be found for using it.... The next important question was how to render the gas portable. This might be accomplished in two ways: By liquefying it, and storing it in lead-lined iron vessels, having a jet with a very fine capillary canal, and fitted with a tap or a screw cap. The tap is turned on, and the cylinder placed in the amount of water required. The chlorine bubbles out, and in ten to fifteen minutes the water is absolutely safe. This method would be of use on a large scale, as for service water carts.”[16]
U.S. Army Major Carl Rogers Darnall, Professor of Chemistry at the Army Medical School, gave the first practical demonstration of this in 1910. Shortly thereafter, Major William J. L. Lyster of the Army Medical Department used a solution of calcium hypochlorite in a linen bag to treat water. For many decades, Lyster's method remained the standard for U.S. ground forces in the field and in camps, implemented in the form of the familiar Lyster Bag (also spelled Lister Bag). This work became the basis for present day systems of municipal water purification.
Sources of water
Further information: Water supply
- Groundwater: The water emerging from some deep ground water may have fallen as rain many tens, hundreds, or thousands of years ago. Soil and rock layers naturally filter the ground water to a high degree of clarity and often it does not require additional treatment other than adding chlorine or chloramines as secondary disinfectants. Such water may emerge as springs, artesian springs, or may be extracted from boreholes or wells. Deep ground water is generally of very high bacteriological quality (i.e., pathogenic bacteria or the pathogenic protozoa are typically absent), but the water may be rich in dissolved solids, especially carbonates and sulfates of calcium and magnesium. Depending on the strata through which the water has flowed, other ions may also be present including chloride, and bicarbonate. There may be a requirement to reduce the iron or manganese content of this water to make it acceptable for drinking, cooking, and laundry use. Primary disinfection may also be required. Where groundwater recharge is practised (a process in which river water is injected into an aquifer to store the water in times of plenty so that it is available in times of drought), the groundwater may require additional treatment depending on applicable state and federal regulations.
- Upland lakes and reservoirs: Typically located in the headwaters of river systems, upland reservoirs are usually sited above any human habitation and may be surrounded by a protective zone to restrict the opportunities for contamination. Bacteria and pathogen levels are usually low, but some bacteria, protozoa or algae will be present. Where uplands are forested or peaty, humic acids can colour the water. Many upland sources have low pH which require adjustment.
- Rivers, canals and low land reservoirs: Low land surface waters will have a significant bacterial load and may also contain algae, suspended solids and a variety of dissolved constituents.
- Atmospheric water generation is a new technology that can provide high quality drinking water by extracting water from the air by cooling the air and thus condensing water vapor.
- Rainwater harvesting or fog collection which collects water from the atmosphere can be used especially in areas with significant dry seasons and in areas which experience fog even when there is little rain.
- Desalination of seawater by distillation or reverse osmosis.
- Surface Water: Freshwater bodies that are open to the atmosphere and are not designated as groundwater are termed surface waters.
Treatment
The processes below are the ones commonly used in water purification plants. Some or most may not be used depending on the scale of the plant and quality of the raw (source) water.Pre-treatment
- Pumping and containment – The majority of water must be pumped from its source or directed into pipes or holding tanks. To avoid adding contaminants to the water, this physical infrastructure must be made from appropriate materials and constructed so that accidental contamination does not occur.
- Screening (see also screen filter) – The first step in purifying surface water is to remove large debris such as sticks, leaves, rubbish and other large particles which may interfere with subsequent purification steps. Most deep groundwater does not need screening before other purification steps.
- Storage – Water from rivers may also be stored in bankside reservoirs for periods between a few days and many months to allow natural biological purification to take place. This is especially important if treatment is by slow sand filters. Storage reservoirs also provide a buffer against short periods of drought or to allow water supply to be maintained during transitory pollution incidents in the source river.
- Pre-chlorination – In many plants the incoming water was chlorinated to minimize the growth of fouling organisms on the pipe-work and tanks. Because of the potential adverse quality effects (see chlorine below), this has largely been discontinued.[17]
pH adjustment
Pure water has a pH close to 7 (neither alkaline nor acidic). Sea water can have pH values that range from 7.5 to 8.4 (moderately alkaline). Fresh water can have widely ranging pH values depending on the geology of the drainage basin or aquifer and the influence of contaminant inputs (acid rain). If the water is acidic (lower than 7), lime, soda ash, or sodium hydroxide can be added to raise the pH during water purification processes. Lime addition increases the calcium ion concentration, thus raising the water hardness. For highly acidic waters, forced draft degasifiers can be an effective way to raise the pH, by stripping dissolved carbon dioxide from the water.[18][19][20] Making the water alkaline helps coagulation and flocculation processes work effectively and also helps to minimize the risk of lead being dissolved from lead pipes and from lead solder in pipe fittings. Sufficient alkalinity also reduces the corrosiveness of water to iron pipes. Acid (carbonic acid, hydrochloric acid or sulfuric acid) may be added to alkaline waters in some circumstances to lower the pH. Alkaline water (above pH 7.0) does not necessarily mean that lead or copper from the plumbing system will not be dissolved into the water. The ability of water to precipitate calcium carbonate to protect metal surfaces and reduce the likelihood of toxic metals being dissolved in water is a function of pH, mineral content, temperature, alkalinity and calcium concentration.[21]Coagulation and flocculation
See also: particle aggregation
One of the first steps in a conventional water purification process
is the addition of chemicals to assist in the removal of particles
suspended in water. Particles can be inorganic such as clay and silt or organic such as algae, bacteria, viruses, protozoa and natural organic matter. Inorganic and organic particles contribute to the turbidity and colour of water.The addition of inorganic coagulants such as aluminum sulfate (or alum) or iron (III) salts such as iron(III) chloride cause several simultaneous chemical and physical interactions on and among the particles. Within seconds, negative charges on the particles are neutralized by inorganic coagulants. Also within seconds, metal hydroxide precipitates of the aluminum and iron (III) ions begin to form. These precipitates combine into larger particles under natural processes such as Brownian motion and through induced mixing which is sometimes referred to as flocculation. The term most often used for the amorphous metal hydroxides is “floc.” Large, amorphous aluminum and iron (III) hydroxides adsorb and enmesh particles in suspension and facilitate the removal of particles by subsequent processes of sedimentation and filtration.[22]:8.2–8.3
Aluminum hydroxides are formed within a fairly narrow pH range, typically: 5.5 to about 7.7. Iron (III) hydroxides can form over a larger pH range including pH levels lower than are effective for alum, typically: 5.0 to 8.5.[23]:679
In the literature, there is much debate and confusion over the usage of the terms coagulation and flocculation—where does coagulation end and flocculation begin? In water purification plants, there is usually a high energy, rapid mix unit process (detention time in seconds) where the coagulant chemicals are added followed by flocculation basins (detention times range from 15 to 45 minutes) where low energy inputs turn large paddles or other gentle mixing devices to enhance the formation of floc. In fact, coagulation and flocculation processes are ongoing once the metal salt coagulants are added.[24]:74–5
Organic polymers were developed in the 1960s as aids to coagulants and, in some cases, as replacements for the inorganic metal salt coagulants. Synthetic organic polymers are high molecular weight compounds that carry negative, positive or neutral charges. When organic polymers are added to water with particulates, the high molecular weight compounds adsorb onto particle surfaces and through interparticle bridging coalesce with other particles to form floc. PolyDADMAC is a popular cationic (positively charged) organic polymer used in water purification plants.[23]:667–8
Sedimentation
Waters exiting the flocculation basin may enter the sedimentation basin, also called a clarifier or settling basin. It is a large tank with low water velocities, allowing floc to settle to the bottom. The sedimentation basin is best located close to the flocculation basin so the transit between the two processes does not permit settlement or floc break up. Sedimentation basins may be rectangular, where water flows from end to end, or circular where flow is from the centre outward. Sedimentation basin outflow is typically over a weir so only a thin top layer of water—that furthest from the sludge—exits.In 1904, Allen Hazen showed that the efficiency of a sedimentation process was a function of the particle settling velocity, the flow through the tank and the surface area of tank. Sedimentation tanks are typically designed within a range of overflow rates of 0.5 to 1.0 gallons per minute per square foot (or 1.25 to 2.5 meters per hour). In general, sedimentation basin efficiency is not a function of detention time or depth of the basin. Although, basin depth must be sufficient so that water currents do not disturb the sludge and settled particle interactions are promoted. As particle concentrations in the settled water increase near the sludge surface on the bottom of the tank, settling velocities can increase due to collisions and agglomeration of particles. Typical detention times for sedimentation vary from 1.5 to 4 hours and basin depths vary from 10 to 15 feet (3 to 4.5 meters).[22]:9.39–9.40[23]:790–1[24]:140–2, 171
Inclined flat plates or tubes can be added to traditional sedimentation basins to improve particle removal performance. Inclined plates and tubes drastically increase the surface area available for particles to be removed in concert with Hazen’s original theory. The amount of ground surface area occupied by a sedimentation basin with inclined plates or tubes can be far smaller than a conventional sedimentation basin.
Sludge storage and removal
As particles settle to the bottom of a sedimentation basin, a layer of sludge is formed on the floor of the tank. This layer of sludge must be removed and treated. The amount of sludge that is generated is significant, often 3 to 5 percent of the total volume of water that is treated. The cost of treating and disposing of the sludge can be a significant part of the operating cost of a water treatment plant. The sedimentation tank may be equipped with mechanical cleaning devices that continually clean the bottom of the tank or the tank can be periodically taken out of service and cleaned manually.Floc blanket clarifiers
A subcategory of sedimentation is the removal of particulates by entrapment in a layer of suspended floc as the water is forced upward. The major advantage of floc blanket clarifiers is that they occupy a smaller footprint than conventional sedimentation. Disadvantages are that particle removal efficiency can be highly variable depending on changes in influent water quality and influent water flow rate.[23]:835–6Dissolved air flotation
When particles to be removed do not settle out of solution easily, dissolved air flotation (DAF) is often used. Water supplies that are particularly vulnerable to unicellular algae blooms and supplies with low turbidity and high colour often employ DAF. After coagulation and flocculation processes, water flows to DAF tanks where air diffusers on the tank bottom create fine bubbles that attach to floc resulting in a floating mass of concentrated floc. The floating floc blanket is removed from the surface and clarified water is withdrawn from the bottom of the DAF tank.[22]:9.46Filtration
After separating most floc, the water is filtered as the final step to remove remaining suspended particles and unsettled floc.Rapid sand filters
The most common type of filter is a rapid sand filter. Water moves vertically through sand which often has a layer of activated carbon or anthracite coal above the sand. The top layer removes organic compounds, which contribute to taste and odour. The space between sand particles is larger than the smallest suspended particles, so simple filtration is not enough. Most particles pass through surface layers but are trapped in pore spaces or adhere to sand particles. Effective filtration extends into the depth of the filter. This property of the filter is key to its operation: if the top layer of sand were to block all the particles, the filter would quickly clog.[25]To clean the filter, water is passed quickly upward through the filter, opposite the normal direction (called backflushing or backwashing) to remove embedded particles. Prior to this step, compressed air may be blown up through the bottom of the filter to break up the compacted filter media to aid the backwashing process; this is known as air scouring. This contaminated water can be disposed of, along with the sludge from the sedimentation basin, or it can be recycled by mixing with the raw water entering the plant although this is often considered poor practice since it re-introduces an elevated concentration of bacteria into the raw water.
Some water treatment plants employ pressure filters. These work on the same principle as rapid gravity filters, differing in that the filter medium is enclosed in a steel vessel and the water is forced through it under pressure.
Advantages:
- Filters out much smaller particles than paper and sand filters can.
- Filters out virtually all particles larger than their specified pore sizes.
- They are quite thin and so liquids flow through them fairly rapidly.
- They are reasonably strong and so can withstand pressure differences across them of typically 2–5 atmospheres.
- They can be cleaned (back flushed) and reused.
Slow sand filters
Slow "artificial" filtration (a variation of bank filtration) to the ground, Water purification plant Káraný, Czech Republic
A specific "large-scale" form of slow sand filter is the process of bank filtration, in which natural sediments in a riverbank are used to provide a first stage of contaminant filtration. While typically not clean enough to be used directly for drinking water, the water gained from the associated extraction wells is much less problematic than river water taken directly from the major streams where bank filtration is often used.
Membrane filtration
Membrane filters are widely used for filtering both drinking water and sewage. For drinking water, membrane filters can remove virtually all particles larger than 0.2 um—including giardia and cryptosporidium. Membrane filters are an effective form of tertiary treatment when it is desired to reuse the water for industry, for limited domestic purposes, or before discharging the water into a river that is used by towns further downstream. They are widely used in industry, particularly for beverage preparation (including bottled water). However no filtration can remove substances that are actually dissolved in the water such as phosphorus, nitrates and heavy metal ions.Removal of ions and other dissolved substances
Ultrafiltration membranes use polymer membranes with chemically formed microscopic pores that can be used to filter out dissolved substances avoiding the use of coagulants. The type of membrane media determines how much pressure is needed to drive the water through and what sizes of micro-organisms can be filtered out.Ion exchange:[26] Ion exchange systems use ion exchange resin- or zeolite-packed columns to replace unwanted ions. The most common case is water softening consisting of removal of Ca2+ and Mg2+ ions replacing them with benign (soap friendly) Na+ or K+ ions. Ion exchange resins are also used to remove toxic ions such as nitrite, lead, mercury, arsenic and many others.
Precipitative softening:[22]:13.12–13.58 Water rich in hardness (calcium and magnesium ions) is treated with lime (calcium oxide) and/or soda-ash (sodium carbonate) to precipitate calcium carbonate out of solution utilizing the common-ion effect.
Electrodeionization:[26] Water is passed between a positive electrode and a negative electrode. Ion exchange membranes allow only positive ions to migrate from the treated water toward the negative electrode and only negative ions toward the positive electrode. High purity deionized water is produced with a little worse degree of purification in comparison with ion exchange treatment. Complete removal of ions from water is regarded as electrodialysis. The water is often pre-treated with a reverse osmosis unit to remove non-ionic organic contaminants.
Disinfection
Disinfection is accomplished both by filtering out harmful micro-organisms and also by adding disinfectant chemicals. Water is disinfected to kill any pathogens which pass through the filters and to provide a residual dose of disinfectant to kill or inactivate potentially harmful micro-organisms in the storage and distribution systems. Possible pathogens include viruses, bacteria, including Salmonella, Cholera, Campylobacter and Shigella, and protozoa, including Giardia lamblia and other cryptosporidia. Following the introduction of any chemical disinfecting agent, the water is usually held in temporary storage – often called a contact tank or clear well to allow the disinfecting action to complete.Chlorine disinfection
Main article: Water chlorination
The most common disinfection method involves some form of chlorine or its compounds such as chloramine or chlorine dioxide. Chlorine is a strong oxidant
that rapidly kills many harmful micro-organisms. Because chlorine is a
toxic gas, there is a danger of a release associated with its use. This
problem is avoided by the use of sodium hypochlorite,
which is a relatively inexpensive solution that releases free chlorine
when dissolved in water. Chlorine solutions can be generated on site by
electrolyzing common salt solutions. A solid form, calcium hypochlorite,
releases chlorine on contact with water. Handling the solid, however,
requires greater routine human contact through opening bags and pouring
than the use of gas cylinders or bleach which are more easily automated.
The generation of liquid sodium hypochlorite is both inexpensive and
safer than the use of gas or solid chlorine.All forms of chlorine are widely used, despite their respective drawbacks. One drawback is that chlorine from any source reacts with natural organic compounds in the water to form potentially harmful chemical by-products. These by-products, trihalomethanes (THMs) and haloacetic acids (HAAs), are both carcinogenic in large quantities and are regulated by the United States Environmental Protection Agency (EPA) and the Drinking Water Inspectorate in the UK. The formation of THMs and haloacetic acids may be minimized by effective removal of as many organics from the water as possible prior to chlorine addition. Although chlorine is effective in killing bacteria, it has limited effectiveness against protozoa that form cysts in water (Giardia lamblia and Cryptosporidium, both of which are pathogenic).
Chlorine dioxide disinfection
Chlorine dioxide is a faster-acting disinfectant than elemental chlorine. It is relatively rarely used, because in some circumstances it may create excessive amounts of chlorite, which is a by-product regulated to low allowable levels in the United States. Chlorine dioxide is supplied as an aqueous solution and added to water to avoid gas handling problems; chlorine dioxide gas accumulations may spontaneously detonate.Chloramine disinfection
The use of chloramine is becoming more common as a disinfectant. Although chloramine is not as strong an oxidant, it does provide a longer-lasting residual than free chlorine and it will not form THMs or haloacetic acids. It is possible to convert chlorine to chloramine by adding ammonia to the water after addition of chlorine. The chlorine and ammonia react to form chloramine. Water distribution systems disinfected with chloramines may experience nitrification, as ammonia is a nutrient for bacterial growth, with nitrates being generated as a by-product.Ozone disinfection
Ozone is an unstable molecule which readily gives up one atom of oxygen providing a powerful oxidizing agent which is toxic to most waterborne organisms. It is a very strong, broad spectrum disinfectant that is widely used in Europe. It is an effective method to inactivate harmful protozoa that form cysts. It also works well against almost all other pathogens. Ozone is made by passing oxygen through ultraviolet light or a "cold" electrical discharge. To use ozone as a disinfectant, it must be created on-site and added to the water by bubble contact. Some of the advantages of ozone include the production of fewer dangerous by-products and the absence of taste and odour problems (in comparison to chlorination) . Although fewer by-products are formed by ozonation, it has been discovered that ozone reacts with bromide ions in water to produce concentrations of the suspected carcinogen bromate. Bromide can be found in fresh water supplies in sufficient concentrations to produce (after ozonation) more than 10 parts per billion (ppb) of bromate — the maximum contaminant level established by the USEPA.[27] Another advantage of ozone is that it leaves no residual disinfectant in the water. Ozone has been used in drinking water plants since 1906 where the first industrial ozonation plant was built in Nice, France. The U.S. Food and Drug Administration has accepted ozone as being safe; and it is applied as an anti-microbiological agent for the treatment, storage, and processing of foods.Ultraviolet disinfection
Ultraviolet light (UV) is very effective at inactivating cysts, in low turbidity water. UV light's disinfection effectiveness decreases as turbidity increases, a result of the absorption, scattering, and shadowing caused by the suspended solids. The main disadvantage to the use of UV radiation is that, like ozone treatment, it leaves no residual disinfectant in the water; therefore, it is sometimes necessary to add a residual disinfectant after the primary disinfection process. This is often done through the addition of chloramines, discussed above as a primary disinfectant. When used in this manner, chloramines provide an effective residual disinfectant with very few of the negative effects of chlorination.Various portable methods of disinfection
Main article: Portable water purification
Available for disinfection in emergencies or in remote locations.
Disinfection is the primary goal, since aesthetic considerations such as
taste, odor, appearance, and trace chemical contamination do not affect
the short-term safety of drinking water.Solar water disinfection
One low-cost method of disinfecting water that can often be implemented with locally available materials is solar disinfection (SODIS).[28][29][30][31][32] Unlike methods that rely on firewood, it has low impact on the environment.One recent study has found that the wild Salmonella which would reproduce quickly during subsequent dark storage of solar-disinfected water could be controlled by the addition of just 10 parts per million of hydrogen peroxide.[33]
Additional treatment options
- Water fluoridation: in many areas fluoride is added to water with the goal of preventing tooth decay.[34] Fluoride is usually added after the disinfection process. In the U.S., fluoridation is usually accomplished by the addition of hexafluorosilicic acid,[35] which decomposes in water, yielding fluoride ions.[36]
- Water conditioning: This is a method of reducing the effects of hard water. In water systems subject to heating hardness salts can be deposited as the decomposition of bicarbonate ions creates carbonate ions that precipitate out of solution. Water with high concentrations of hardness salts can be treated with soda ash (sodium carbonate) which precipitates out the excess salts, through the common-ion effect, producing calcium carbonate of very high purity. The precipitated calcium carbonate is traditionally sold to the manufacturers of toothpaste. Several other methods of industrial and residential water treatment are claimed (without general scientific acceptance) to include the use of magnetic and/or electrical fields reducing the effects of hard water.[citation needed]
- Plumbosolvency reduction: In areas with naturally acidic waters of low conductivity (i.e. surface rainfall in upland mountains of igneous rocks), the water may be capable of dissolving lead from any lead pipes that it is carried in. The addition of small quantities of phosphate ion and increasing the pH slightly both assist in greatly reducing plumbo-solvency by creating insoluble lead salts on the inner surfaces of the pipes.
- Radium Removal: Some groundwater sources contain radium, a radioactive chemical element. Typical sources include many groundwater sources north of the Illinois River in Illinois. Radium can be removed by ion exchange, or by water conditioning. The back flush or sludge that is produced is, however, a low-level radioactive waste.
- Fluoride Removal: Although fluoride is added to water in many areas, some areas of the world have excessive levels of natural fluoride in the source water. Excessive levels can be toxic or cause undesirable cosmetic effects such as staining of teeth. Methods of reducing fluoride levels is through treatment with activated alumina and bone char filter media.
Other water purification techniques
Other popular methods for purifying water, especially for local private supplies are listed below. In some countries some of these methods are also used for large scale municipal supplies. Particularly important are distillation (de-salination of seawater) and reverse osmosis.- Boiling: Bringing it to its boiling point at 100 °C (212 °F), is the oldest and most effective way since it eliminates most microbes causing intestine related diseases,[37] but it cannot remove chemical toxins or impurities.[38] For human health, complete sterilization of water is not required, since the heat resistant microbes are not intestine affecting.[37] The traditional advice of boiling water for ten minutes is mainly for additional safety, since microbes start getting eliminated at temperatures greater than 60 °C (140 °F). Though the boiling point decreases with increasing altitude, it is not enough to affect the disinfecting process.[37][39] In areas where the water is "hard" (that is, containing significant dissolved calcium salts), boiling decomposes the bicarbonate ions, resulting in partial precipitation as calcium carbonate. This is the "fur" that builds up on kettle elements, etc., in hard water areas. With the exception of calcium, boiling does not remove solutes of higher boiling point than water and in fact increases their concentration (due to some water being lost as vapour). Boiling does not leave a residual disinfectant in the water. Therefore, water that is boiled and then stored for any length of time may acquire new pathogens.
- Granular Activated Carbon filtering: a form of activated carbon with a high surface area, adsorbs many compounds including many toxic compounds. Water passing through activated carbon is commonly used in municipal regions with organic contamination, taste or odors. Many household water filters and fish tanks use activated carbon filters to further purify the water. Household filters for drinking water sometimes contain silver as metallic silver nanoparticle. If water is held in the carbon block for longer period, microorganisms can grow inside which results in fouling and contamination. Silver nanoparticles are excellent anti-bacterial material and they can decompose toxic halo-organic compounds such as pesticides into non-toxic organic products.[40]
- Distillation involves boiling the water to produce water vapour. The vapour contacts a cool surface where it condenses as a liquid. Because the solutes are not normally vaporised, they remain in the boiling solution. Even distillation does not completely purify water, because of contaminants with similar boiling points and droplets of unvapourised liquid carried with the steam. However, 99.9% pure water can be obtained by distillation.
- Reverse osmosis: Mechanical pressure is applied to an impure solution to force pure water through a semi-permeable membrane. Reverse osmosis is theoretically the most thorough method of large scale water purification available, although perfect semi-permeable membranes are difficult to create. Unless membranes are well-maintained, algae and other life forms can colonize the membranes.
- The use of iron in removing arsenic from water. See Arsenic contamination of groundwater.
- Direct contact membrane distillation (DCMD). Applicable to desalination. Heated seawater is passed along the surface of a hydrophobic polymer membrane. Evaporated water passes from the hot side through pores in the membrane into a stream of cold pure water on the other side. The difference in vapour pressure between the hot and cold side helps to push water molecules through.
- Desalination – is a process by which saline water (generally sea water) is converted to fresh water. The most common desalination processes are distillation and reverse osmosis. Desalination is currently expensive compared to most alternative sources of water, and only a very small fraction of total human use is satisfied by desalination. It is only economically practical for high-valued uses (such as household and industrial uses) in arid areas.
- Gas hydrate crystals centrifuge method. If carbon dioxide or other low molecular weight gas is mixed with contaminated water at high pressure and low temperature, gas hydrate crystals will form exothermically. Separation of the crystalline hydrate may be performed by centrifuge or sedimentation and decanting. Water can be released from the hydrate crystals by heating[41]
- In Situ Chemical Oxidation, a form of advanced oxidation processes and advanced oxidation technology, is an environmental remediation technique used for soil and/or groundwater remediation to reduce the concentrations of targeted environmental contaminants to acceptable levels. ISCO is accomplished by injecting or otherwise introducing strong chemical oxidizers directly into the contaminated medium (soil or groundwater) to destroy chemical contaminants in place. It can be used to remediate a variety of organic compounds, including some that are resistant to natural degradation
Safety and controversies
Further information: Distilled water#Health concerns
Drinking water pollution detector Rainbow trout (Oncorhynchus mykiss) are being used in water purification plants to detect acute water pollution
|
|
The examples and perspective in this article deal primarily with the United States and do not represent a worldwide view of the subject. Please improve this article and discuss the issue on the talk page. (April 2011) |
Many municipalities have moved from free chlorine to chloramine as a disinfection agent. However, chloramine appears to be a corrosive agent in some water systems. Chloramine can dissolve the "protective" film inside older service lines, leading to the leaching of lead into residential spigots. This can result in harmful exposure, including elevated blood lead levels. Lead is a known neurotoxin.[43]
Demineralized water
Distillation removes all minerals from water, and the membrane methods of reverse osmosis and nanofiltration remove most to all minerals. This results in demineralized water which is not considered ideal drinking water. The World Health Organization has investigated the health effects of demineralized water since 1980.[44] Experiments in humans found that demineralized water increased diuresis and the elimination of electrolytes, with decreased blood serum potassium concentration. Magnesium, calcium, and other minerals in water can help to protect against nutritional deficiency. Demineralized water may also increase the risk from toxic metals because it more readily leaches materials from piping like lead and cadmium, which is prevented by dissolved minerals such as calcium and magnesium. Low-mineral water has been implicated in specific cases of lead poisoning in infants, when lead from pipes leached at especially high rates into the water. Recommendations for magnesium have been put at a minimum of 10 mg/L with 20–30 mg/L optimum; for calcium a 20 mg/L minimum and a 40–80 mg/L optimum, and a total water hardness (adding magnesium and calcium) of 2 to 4 mmol/L. At water hardness above 5 mmol/L, higher incidence of gallstones, kidney stones, urinary stones, arthrosis, and arthropathies have been observed.[45] Additionally, desalination processes can increase the risk of bacterial contamination.[45]Manufacturers of home water distillers claim the opposite—that minerals in water are the cause of many diseases, and that most beneficial minerals come from food, not water.[46][47] They quote the American Medical Association as saying "The body's need for minerals is largely met through foods, not drinking water." The WHO report agrees that "drinking water, with some rare exceptions, is not the major source of essential elements for humans" and is "not the major source of our calcium and magnesium intake", yet states that demineralized water is harmful anyway. "Additional evidence comes from animal experiments and clinical observations in several countries. Animals given zinc or magnesium dosed in their drinking water had a significantly higher concentration of these elements in the serum than animals given the same elements in much higher amounts with food and provided with low-mineral water to drink."
See also
|
|
This "see also" section may contain an excessive number of suggestions. Please ensure that only the most relevant suggestions are given and that they are not red links, and consider integrating suggestions into the article itself. (June 2013) |
- Aquatic toxicology
- American Water Works Association
- Bacteriological water analysis
- Carl Rogers Darnall
- Ecological sanitation
- Environmental Engineering
- Environmental Engineering Science
- Environmental Persistent Pharmaceutical Pollutant EPPP
- ERDLator Was the name for the 1940s–1950s "Water Purification Unit, Van-Type, Body Mounted, Electric Driven.
- List of waste water treatment technologies
- Microfiltration
- National Rural Water Association
- Organisms used in water purification
- Sewage treatment
- Swimming pool sanitation
- The Environmental Institute
- Treatment pond
- Water conservation
- Water recycling
- Water treatment
References
- Jump up ^ Combating Waterborne Diseases at the Household Level (PDF). World Health Organization. 2007. Part 1. ISBN 978-92-4-159522-3.
- Jump up ^ Water for Life: Making it Happen (PDF). World Health Organization and UNICEF. 2005. ISBN 92-4-156293-5.
- Jump up ^ "The Use of the Microscope in Water Filter History". History of Water Filters.
- ^ Jump up to: a b Filtration of water supplies, World Health Organization
- Jump up ^ History of the Chelsea Waterworks
- Jump up ^ Gunn, S. William A. and Masellis, Michele (2007). Concepts and Practice of Humanitarian Medicine. Springer. p. 87. ISBN 978-0-387-72264-1.
- Jump up ^ Bazin, Hervé (2008). L'histoire des vaccinations. John Libbey Eurotext. p. 290.
- Jump up ^ An Act to make better Provision respecting the Supply of Water to the Metropolis, (15 & 16 Vict. C.84)
- Jump up ^ Turneaure, F.E., and H.L. Russell (1901). Public Water-Supplies: Requirements, Resources, and the Construction of Works (1st ed.). New York: John Wiley & Sons. p. 493.
- Jump up ^ "Typhoid Epidemic at Maidstone". Journal of the Sanitary Institute 18: 388. October 1897.
- Jump up ^ "A miracle for public health?". Retrieved 2012-12-17.
- Jump up ^ Reece, R.J. (1907). “Report on the Epidemic of Enteric Fever in the City of Lincoln, 1904-5.” In Thirty-Fifth Annual Report of the Local Government Board, 1905-6: Supplement Containing the Report of the Medical Officer for 1905-6. London:Local Government Board.
- Jump up ^ Leal, John L. (1909). “The Sterilization Plant of the Jersey City Water Supply Company at Boonton, N.J.” Proceedings American Water Works Association. pp. 100–9.
- Jump up ^ Fuller, George W. (1909). “Description of the Process and Plant of the Jersey City Water Supply Company for the Sterilization of the Water of the Boonton Reservoir.” Proceedings AWWA. pp. 110–34.
- Jump up ^ Hazen, Allen. (1916). Clean Water and How to Get It. New York:Wiley. p. 102.
- Jump up ^ Nesfield, V. B. (1902). "A Chemical Method of Sterilizing Water Without Affecting its Potability". Public Health: 601–3.
- Jump up ^ Chen, Jimmy, and Regli, Stig. (2002). "Disinfection Practices and Pathogen Inactivation in ICR Surface Water Plants." Information Collection Rule Data Analysis. Denver:American Water Works Association. McGuire, Michael J., McLain, Jennifer L. and Obolensky, Alexa, eds. pp. 376–378. ISBN 1-58321-273-6
- Jump up ^ Aeration and Gas Stripping, Accessed June 4, 2012.
- Jump up ^ CO2 Degasifiers/Drinking Water Corrosion Control, tudelft.nl.
- Jump up ^ Degassing Towers, forbesgroup.co.uk.
- Jump up ^ RTW corrosivity index calculator, American Water Works Association .
- ^ Jump up to: a b c d Edzwald, James K., ed. (2011). Water Quality and Treatment. 6th Edition. New York:McGraw-Hill. ISBN 978-0-07-163011-5
- ^ Jump up to: a b c d Crittenden, John C., et al., eds. (2005). Water Treatment: Principles and Design. 2nd Edition. Hoboken, NJ:Wiley. ISBN 0-471-11018-3
- ^ Jump up to: a b Kawamura, Susumu. (2000). Integrated Design and Operation of Water Treatment Facilities. 2nd Edition. New York:Wiley. pp. 74–5, 104. ISBN 0-471-35093-1
- Jump up ^ United States Environmental Protection Agency (EPA)(1990). Cincinnati, OH. "Technologies for Upgrading Existing or Designing New Drinking Water Treatment Facilities." Document no. EPA/625/4-89/023.
- ^ Jump up to: a b Andrei A. Zagorodni (2007). Ion exchange materials: properties and applications. Elsevier. ISBN 978-0-08-044552-6.
- Jump up ^ Neemann, Jeff; Hulsey, Robert; Rexing, David; Wert, Eric (2004). "Controlling Bromate Formation During Ozonation with Chlorine and Ammonia". Journal American Water Works Association 96 (2): 26–29.
- Jump up ^ Conroy RM, Meegan ME, Joyce T, McGuigan K, Barnes J (October 1999). "Solar disinfection of water reduces diarrhoeal disease, an update". Arch Dis Child 81 (4): 337–8. doi:10.1136/adc.81.4.337. PMC 1718112. PMID 10490440.
- Jump up ^ Conroy RM, Meegan ME, Joyce TM, McGuigan KG, Barnes J (2001). "Use of solar disinfection protects children under 6 years from cholera". Arch Dis Child 85 (4): 293–5. doi:10.1136/adc.85.4.293. PMC 1718943. PMID 11567937.
- Jump up ^ Rose A. at al. (February 2006). "Solar disinfection of water for diarrhoeal prevention in southern India". Arch Dis Child 91 (2): 139–41. doi:10.1136/adc.2005.077867. PMC 2082686. PMID 16403847.
- Jump up ^ Hobbins M. (2003). The SODIS Health Impact Study, Ph.D. Thesis, Swiss Tropical Institute Basel
- Jump up ^ Dawney, B. and Pearce, J.M. (2012). "Optimizing Solar Water Disinfection (SODIS) Method by Decreasing Turbidity with NaCl". The Journal of Water, Sanitation, and Hygiene for Development 2 (2): 87–94.
- Jump up ^ Sciacca F, Rengifo-Herrera JA, Wéthé J, Pulgarin C (2010-01-08). "Dramatic enhancement of solar disinfection (SODIS) of wild Salmonella sp. in PET bottles by H(2)O(2) addition on natural water of Burkina Faso containing dissolved iron". Chemosphere (epub ahead of print) 78 (9): 1186–91. doi:10.1016/j.chemosphere.2009.12.001. PMID 20060566.
- Jump up ^ Centers for Disease Control and Prevention (2001). "Recommendations for using fluoride to prevent and control dental decay caries in the United States". MMWR Recomm Rep 50 (RR-14): 1–42. PMID 11521913. Lay summary – CDC (2007-08-09).
- Jump up ^ Division of Oral Health, National Center for Prevention Services, CDC (1993). Fluoridation census 1992 (PDF). Retrieved 2008-12-29.
- Jump up ^ Reeves TG (1986). "Water fluoridation: a manual for engineers and technicians" (PDF). Centers for Disease Control. Retrieved 2008-12-10.
- ^ Jump up to: a b c Backer, Howard (2002). "Water Disinfection for International and Wilderness Travelers". Clin Infect Dis. 34 (3): 355–364. doi:10.1086/324747.
- Jump up ^ Curtis, Rick (1998) OA Guide to Water Purification, The Backpacker's Field Manual, Random House.
- Jump up ^ "Is it true that you can't make a decent cup of tea up a mountain?". physics.org. Retrieved 2 November 2012.
- Jump up ^ Savage, Nora; Mamadou S. Diallo (2005). "Nanomaterials and Water Purification: Opportunities and Challenges". J. Nanopart. Res. 7 (4–5): 331–342. doi:10.1007/s11051-005-7523-5. Retrieved 24 May 2011.
- Jump up ^ Osegovic, John P. et al. (2009) Hydrates for Gypsum Stack Water Purification. AIChE Annual Convention
- Jump up ^ Poulsen, Kevin (26 April 2007). "Mysterious Glitch Poisons Town Water Supply". Wired.
- Jump up ^ Miranda, M. L.; Kim, D.; Hull, A. P.; Paul, C. J.; Galeano, M. A. O. (2006). "Changes in Blood Lead Levels Associated with Use of Chloramines in Water Treatment Systems". Environmental Health Perspectives 115 (2): 221–225. doi:10.1289/ehp.9432. PMC 1817676. PMID 17384768. edit
- Jump up ^ Health risks from drinking demineralised water. (PDF) . Rolling revision of the WHO Guidelines for drinking-water quality. World Health Organization, Geneva, 2004
- ^ Jump up to: a b Kozisek F. (2004). Health risks from drinking demineralised water. WHO.
- Jump up ^ Water Distillers – Water Distillation – Myths, Facts, etc. Naturalsolutions1.com. Retrieved on 2011-02-18.
- Jump up ^ Minerals in Drinking Water. Aquatechnology.net. Retrieved on 2011-02-18.
Further reading
- Standard Methods for the Examination of Water & Wastewater. American Public Health Association. ISBN 0-87553-047-8.
- Masters, Gilbert M. Introduction to Environmental Engineering. 2nd ed. Upper Saddle River, NJ: Prentice Hall, 1998.
- US EPA. "Ground Water and Drinking Water." Overview of drinking water topics and detailed information on US regulatory program. (Updated 2012-03-07.)
External links
| Wikimedia Commons has media related to Water_purification. |
- EPA. "Water On Tap: What You Need To Know." – Consumer Guide to Drinking Water in the US
- CDC Emergency Disinfection of Drinking Water- Camping, Hiking and Travel
- Code of Federal Regulations, Title 40, Part 141 – U.S. National Primary Drinking Water Regulations
- Code of Federal Regulations, Title 21, Part 129 – U.S. Food and Drug Administration regulations on bottled water
|
||||||||||||||||
Navigation menu
- This page was last modified on 23 February 2014 at 23:32.
- Text is available under the Creative Commons Attribution-ShareAlike License;
additional terms may apply. By using this site, you agree to the Terms of Use and Privacy Policy.
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a non-profit organization.
Subscribe to:
Comments (Atom)
-
Heavy Metals S 31 HEAVY METALS This test is provided to demonstrate that the content of metallic impurities...
-
Instrumentation HPLC systems HPLC instrumentation includes a pump, injector, column, detector and recorder or data system, connected as...

